Book traversal links for 1266
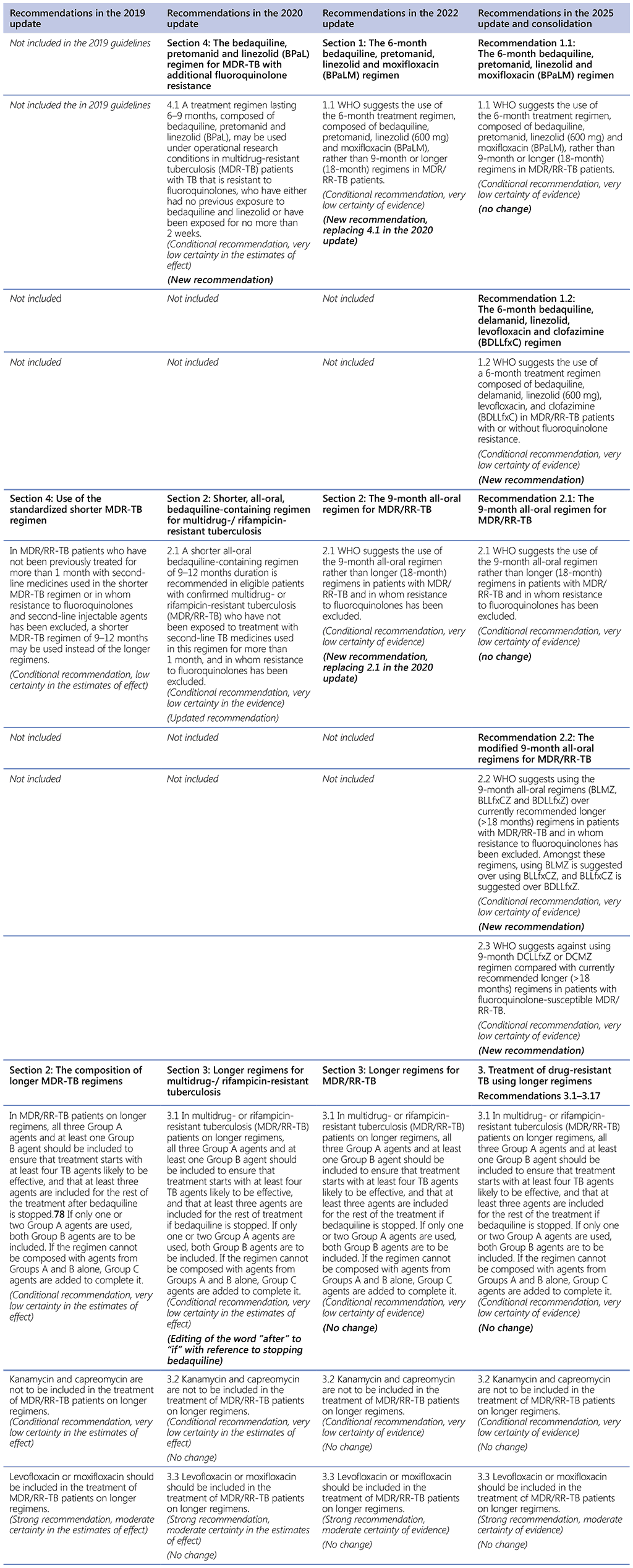
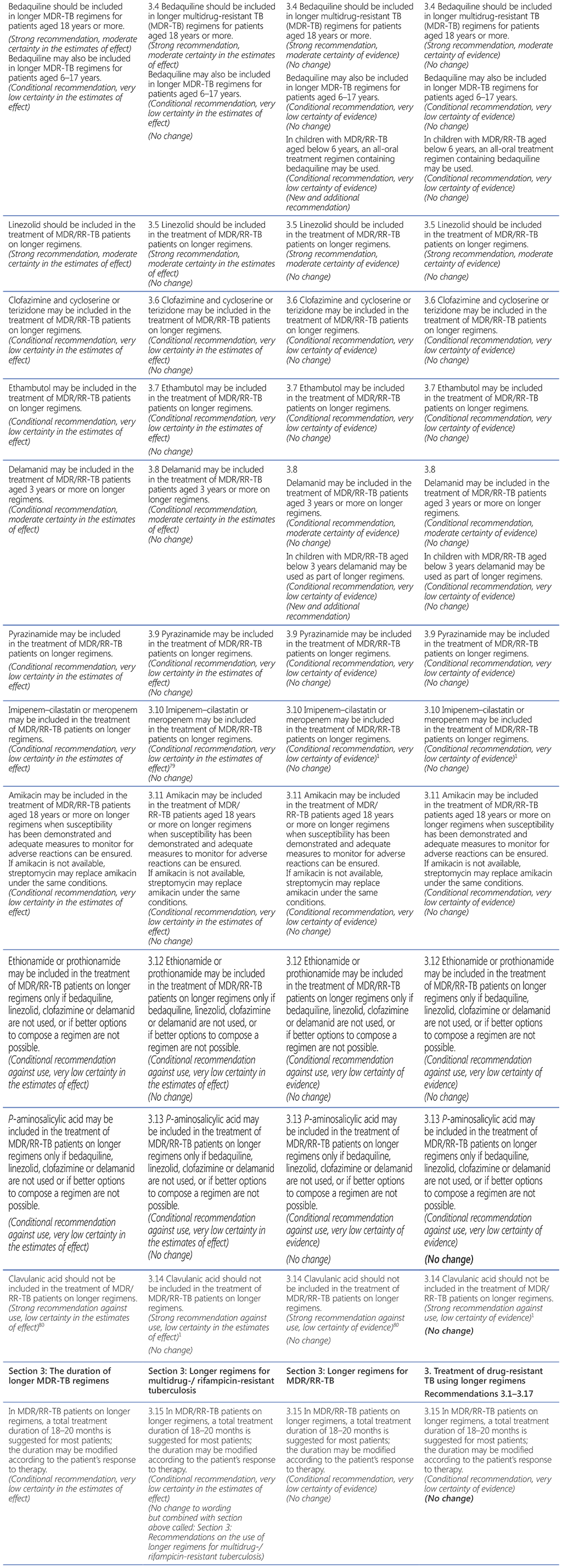

78 Group A = levofloxacin/moxifloxacin, bedaquiline, linezolid; Group B = clofazimine, cycloserine/terizidone; Group C = ethambutol, delamanid, pyrazinamide, imipenem–cilastatin, meropenem, amikacin (streptomycin), ethionamide/prothionamide, p-aminosalicylic acid (see also Table 3.1).
79 Imipenem–cilastatin and meropenem are administered with clavulanic acid, which is available only in formulations combined with amoxicillin. Amoxicillin–clavulanic acid is not counted as an additional effective TB agent, and should not be used without imipenem– cilastatin or meropenem.
80 Imipenem–cilastatin and meropenem are administered with clavulanic acid, which is available only in formulations combined with amoxicillin (amoxicillin–clavulanic acid). When included, clavulanic acid is not counted as an additional effective TB agent and should not be used without imipenem–cilastatin or meropenem.

 Feedback
Feedback