Book traversal links for 1266
Recommendation 2.1 The 9-month all-oral regimen for MDR/RR-TB

Remarks
- The 9-month all-oral regimen consists of bedaquiline (used for 6 months), in combination with levofloxacin/moxifloxacin, ethionamide, ethambutol, isoniazid (high-dose), pyrazinamide and clofazimine (for 4 months, with the possibility of extending to 6 months if the patient remains sputum smear positive at the end of 4 months), followed by treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide (for 5 months). Ethionamide can be replaced by 2 months of linezolid (600 mg daily).
- A 9-month regimen with linezolid instead of ethionamide may be used in pregnant women, unlike the regimen with ethionamide.
- This recommendation applies to:
- people with MDR/RR-TB and without resistance to fluoroquinolones;
- patients without extensive TB disease³² and without severe extrapulmonary TB;³³
- patients with less than 1 month exposure to bedaquiline, fluoroquinolones, ethionamide, linezolid and clofazimine; when exposure is greater than 1 month, these patients may still receive this regimen if resistance to the specific medicines with such exposure has been ruled out;
- all people regardless of HIV status;
- children (and patients in other age groups) who do not have bacteriological confirmation of TB or resistance patterns but who do have a high likelihood of MDR/RR-TB (based on clinical signs and symptoms of TB, in combination with a history of contact with a patient with confirmed MDR/RR-TB).
Rationale
The rationale for this recommendation is based on the evidence and considerations described in detail in the following two subsections. The 9-month regimens can be used in patients not eligible for the shorter, 6-month regimens; also, they represent a preferred treatment option over the longer regimens. The intention to determine a relatively shorter duration of treatment for patients with forms of DR-TB or other eligibility criteria not compatible with the 6-month regimen has driven the assessments presented in this section.
Briefly, two assessments have been performed: first, comparing the outcomes of the 9-month regimen including linezolid for 2 months and the identical regimen that included ethionamide for 4 months; and second, comparing the outcomes of the 9-month regimen including linezolid with the longer regimens that were designed individually but included both bedaquiline and linezolid along with other medicines as recommended by WHO. Data on most of the 9-month regimens were obtained from a programmatic setting in South Africa.
The first assessment showed similar levels of treatment success (64% vs 66%), failure or recurrence (1.1% vs 1.4%), deaths (20% vs 21%), loss to follow-up (15% vs 12%) and amplification of drug resistance (0.6% vs 0%). AEs were noted in 5% of participants receiving the 9-month regimen with linezolid; however, no comparisons could be made because no data were available for participants receiving the 9-month regimen with ethionamide. The second assessment of the 9-month regimen compared with longer regimens also showed lower levels of treatment success (64% vs 74%), failure or recurrence (1% vs 3%) or amplification of drug resistance (1% vs 2%); and higher levels of deaths (20% vs 11%) or loss to follow-up (15% vs 12%). AEs were noted in 5% of participants receiving the 9-month regimen with linezolid and in participants receiving longer regimens.
Based on a combined review of these two assessments it was considered that the 9-month regimen with linezolid can be recommended as an alternative to the 9-month regimen with ethionamide, and that both regimens can be used in preference to the longer (18-month) regimens in eligible patients. These assessments were performed on the background of the previous assessment during the GDG meeting in 2019 that led to the conditional recommendation for use of the 9-month alloral bedaquiline-containing regimen (29). The datasets of both 9-month regimens systematically excluded patients with extensive TB disease and severe forms of extrapulmonary TB; therefore, this recommendation is not extended to these groups of patients.
Summary of evidence
This section provides the PICO questions posed, the data and studies considered to answer the questions, the methods used for analysis and data synthesis, a summary of evidence on desirable and undesirable effects and certainty of evidence, and a summary of other evidence considered during development of the recommendation. Additional detail on the evidence is available in the annexes containing the GRADE evidence summary tables and GRADE evidence-to-decision tables (Annex 5).
PICO questions
The following PICO question was used for the evidence assessment in 2019 that led to the conditional recommendation for use of the all-oral bedaquiline-containing 9-month regimen:
PICO question 2–2019 (MDR/RR-TB, 2019): In MDR/RR-TB patients, does an all-oral treatment regimen lasting 9–12 months and including bedaquiline safely improve outcomes when compared with other regimens conforming to WHO guidelines?
The following PICO question (split into two sub-PICO questions because of different comparators) guided the analyses and the assessment, and eventually led to a summary recommendation:
PICO question 1–2022 (MDR/RR-TB, 2022): Should a shorter all-oral regimen (less than 12 months) containing at least three Group A medicines³⁴ be used in patients with MDR/RR-TB and with fluoroquinolone resistance excluded?
Data and studies considered
In 2019, for the WHO guideline update, the South African Department of Health provided WHO with access to programmatic data on injectable-free regimens that had been used in South Africa since 2017, when most eligible patients were enrolled on a shorter regimen, with bedaquiline replacing the injectable (53). In August 2019, WHO issued a public call for IPD on the use of all-oral shorter regimens of 9–12 months (54), but this call yielded no additional evidence on the implementation of such regimens. Consequently, the evidence review on injectable-free regimens in 2019 was based primarily on programmatic data from South Africa, recorded in the Electronic Drug-Resistant Tuberculosis Register (EDRWeb). Secondary comparative analyses were carried out using the IPD, to balance the assumptions and adequacy of the data, and adding to the generalizability of findings – in particular, the applicability to a global population. The IPD used at that time was a global dataset of the records of individual patients who have been treated for MDR/RR-TB; as of November 2019, it contained 13 273 records from 55 studies or centres in 38 countries. The evidence reviews focused on the performance of a standardized shorter regimen in which the injectable agent was replaced by bedaquiline, in combination with levofloxacin (or moxifloxacin), clofazimine, and high-dose isoniazid, ethambutol, pyrazinamide and ethionamide (or prothionamide). Patients on this regimen did not receive any injectable agents, nor were they administered cycloserine, terizidone, p-aminosalicylic acid, delamanid or linezolid. According to the clinical guidance issued by the South African Department of Health, at the time of regimen roll-out patients were not enrolled on the all-oral shorter regimen if they had extensive disease, severe extrapulmonary TB, fluoroquinolone resistance, previous exposure to second-line treatment for more than 1 month or genotypic DST showing mutations in both inhA and katG genes.
In June 2021, WHO issued a public call (55) for IPD on the treatment of DR-TB. The call for individual patients’ data on bacteriologically confirmed MDR/RR-TB patients (including MDR/RR-TB, MDR/RR-TB with additional resistance to second-line TB drugs, and patients with pre-XDR-TB or XDR-TB) included the following specifics:
- use of the modified shorter (<12 months) all-oral regimens using at least bedaquiline and linezolid;
- use of the WHO-recommended shorter all-oral bedaquiline-containing regimen (9–11 months) in the following combination: 4 or 6 months of bedaquiline (used for at least 6 months), levofloxacin (or moxifloxacin), clofazimine, pyrazinamide, ethionamide, ethambutol and high-dose isoniazid, followed by 5 months of levofloxacin (or moxifloxacin), clofazimine, pyrazinamide and ethambutol; and
- use of the WHO-recommended longer all-oral treatment regimen containing at least bedaquiline and linezolid.
The South African Department of Health provided WHO with the programmatic data from 2018 to 2019 on the use of a 9-month regimen in which ethionamide was replaced by linezolid. Several country programmes that provided WHO with IPD on the use of longer regimens according to WHO recommendations are listed in the Introduction (See Scope of the 2022 update and available evidence).
Once again, in 2021, the evidence review was based on programmatic data from South Africa on treatment outcomes of patients treated with the 9-month regimen (with either ethionamide or linezolid), recorded in the EDRWeb. Both datasets from South Africa (2017 and 2018–2019) with the 9-month regimens systematically excluded patients with extensive TB disease (extensive bilateral pulmonary cavitations), severe forms of extrapulmonary TB (meningoencephalitis, osteoarticular TB, pericardial effusion), fluoroquinolone resistance, previous exposure to second-line treatment for more than 1 month or with genotypic DST showing mutations in both inhA and katG genes. In addition, comparative analyses were carried out using the 2021 IPD, which was compiled for the review and analyses in preparation for the GDG 2022; this IPD was of individual patients who had been treated for MDR/RR-TB. The evidence review focused on the performance of a standardized shorter regimen in which the injectable agent was replaced by bedaquiline (used for 6 months), in combination with levofloxacin/moxifloxacin, ethionamide, ethambutol, isoniazid (high-dose), pyrazinamide and clofazimine for 4 months (with the possibility of extending to 6 months if the patient remained sputum smear positive at the end of 4 months), followed by 5 months of treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide. The comparators used included a nearly identical regimen where ethionamide was replaced by 2 months of linezolid (600 mg once daily) and the set of longer regimens designed based on the 2020 WHO recommendations.
Methods used for analysis and data synthesis
For comparisons between dataset or cohorts, outcomes were presented as unadjusted RRs and aRRs; the latter were calculated using a log-binomial generalized linear regression (binomial error distribution with log link function). Confounders were adjusted for using inverse probability propensity score weighting. No convergence issues with the log-binomial model arose. When outcome rates were close to the boundary (<5 positive or negative cases) aRRs were not calculated and unadjusted RRs alone were presented. For outcomes where the number of outcome events was zero, an unadjusted RD was calculated. For unadjusted RDs or RRs, the score method was used for calculating CIs. These approaches applied where one arm of a randomized trial was being compared with an external population, and in randomized trials in which subgroup analyses were performed (including by fluoroquinolone resistance status). Covariate selection for calculation of propensity scores was based on data availability and clinical knowledge. The covariates considered for inclusion in the propensity scores analysis included age, gender, baseline smear result, HIV status (including antiretroviral treatment status), prior treatment history (including whether previous infection was drug resistant), body mass index, smoking status, diabetes diagnosis, cavitation at baseline, disease site and presence of bilateral disease. For the calculation of aRRs, multiple imputation by chain equations using the “within” propensity score approach was used to account for missing data in potential confounders when the proportion of missing values for a confounder was less than 45%.
Summary of evidence on desirable and undesirable effects and certainty of evidence
PICO 1–2019
The primary analysis performed in 2019 using programmatic data from South Africa indicated that the use of a shorter all-oral bedaquiline-containing regimen in patients with MDR/RR-TB was associated with:
- higher treatment success rates (73% all-oral versus 60% standardized shorter regimen success rates, adjusted odds ratio [aOR] for success versus failure or recurrence: 2.1, 95% CI: 1.1–4.0; aOR success versus death: 1.6, 95% CI: 1.2–2.1; aOR success versus failure, recurrence or death: 1.7, 95% CI: 1.3–2.2; and aOR success versus all unfavourable outcomes: 1.9, 95% CI: 1.6–2.4); and
- lower loss to follow-up than a standardized shorter regimen in which an injectable agent was used (aOR loss to follow-up versus all other outcomes: 0.5, 95% CI: 0.4–0.7).
A similar effect for subgroups of patients with acid-fast bacilli (AFB) smear-positive sputum and PLHIV and HIV-negative patients was observed with the use of the shorter all-oral bedaquiline-containing regimen.
The analysis also indicated that when the shorter all-oral bedaquiline-containing regimen was compared with an injectable-free longer regimen containing bedaquiline, there seemed to be no marked differences in the outcomes observed. However, relatively modest beneficial effects were noted in the direction of the intervention; in particular, success versus failure or recurrence (aOR: 3.9, 95% CI: 1.7–9.1), success versus all unfavourable outcomes (aOR: 1.6, 95% CI: 1.2–2.2) and loss to follow-up (aOR: 0.5, 95% CI: 0.4–0.8), all favouring the use of the all-oral shorter regimen. Further subgroup analysis suggested consistent differences in treatment outcomes, as observed in primary analyses among subgroups, in particular among AFB smear-positive patients and in PLHIV on ART; however, differences in treatment outcomes in all-oral shorter and longer regimens were no longer significant when looking at outcomes for HIV-negative individuals, with the exception of loss to follow-up, which favoured the intervention. The additional comparison also illustrated the effect of a shorter all-oral bedaquiline-containing regimen in comparison with longer regimens without any new drugs.The all-oral shorter regimen performed significantly better across all outcomes and all subgroups in this comparison.
PICO 1–2022
For the assessment performed in preparation for the 2022 GDG, 8653 records of patients with MDR/RR-TB initiating TB treatment at any time between January and December 2017 were considered, of which the following were included for analyses: 4244 patients treated with a shorter regimen that included linezolid (used in South Africa in 2019) (intervention), 880 patients who received a shorter all-oral bedaquiline-containing 9-month regimen with ethionamide (used in South Africa in 2017) (comparator), and 850 patients treated with longer regimens that included at least bedaquiline and linezolid.
Sub-PICO 1.1
In sub-PICO 1.1, two observational studies were compared – the 9-month regimen with linezolid (used in South Africa in 2019) (intervention) and the 9-month regimen with ethionamide (used in South Africa in 2017) (comparator). Both datasets were obtained from a programmatic setting in South Africa.
Participants with MDR/RR-TB with fluoroquinolone susceptibility receiving the 9-month regimen with linezolid (n=4244) compared with participants receiving the 9-month regimen with ethionamide (n=880) experienced:
- lower levels of treatment success (64% vs 66%); that is, a 4% relative reduction (aRR=0.96, 95% CI: 0.91 to 1.01);
- lower levels of failure and recurrence (1.1% vs 1.4%); that is, a 20% relative reduction (aRR=0.80, 95% CI: 0.42 to 1.53);
- higher levels of deaths (20% vs 21%); that is, a 3% relative increase (aRR=1.03, 95% CI: 0.89 to 1.20)³⁵
- higher levels of loss to follow-up (15% vs 12%); that is, a 19% relative increase (aRR=1.19, 95% CI: 0.98 to 1.45); and
- higher levels of amplification of drug resistance (0.6% vs 0%); that is, a 1% absolute increase (RD=0.01, 95% CI: 0.00 to 0.01).
AEs were noted in 5% of participants receiving the 9-month regimen with linezolid but no comparisons could be made because no data were available for participants receiving the 9-month regimen with ethionamide.
The GDG judged the benefits of the 9-month regimen with linezolid to be small and the undesirable effects to be moderate compared with the 9-month regimen with ethionamide. The certainty of evidence was judged to be very low. Based on this, the GDG judged that the balance of health effects does not favour either the 9-month regimen with linezolid or the 9-month regimen with ethionamide.
Conclusion
The use of either the 9-month regimen with linezolid or the 9-month regimen with ethionamide is suggested in people with pulmonary MDR/RR-TB without fluoroquinolone resistance (conditional recommendation, very low certainty of evidence).
Sub-PICO 1.2
In sub-PICO 1.2, two observational datasets were compared – the 9-month regimen with linezolid (used in South Africa in 2019) (intervention) and the all-oral longer regimens containing bedaquiline from the 2021 IPD dataset.
Participants with MDR/RR-TB with fluoroquinolone susceptibility receiving the 9-month regimen with linezolid (n=4244) compared with participants receiving longer regimens for MDR/RR-TB (n=850) experienced:
- lower levels of treatment success (64% vs 74%); that is, a 10% relative reduction (aRR=0.90, 95% CI: 0.83 to 0.98);
- lower levels of failure and recurrence (1.1% vs 3.4%); that is, a 71% relative reduction (aRR=0.29, 95% CI: 0.14 to 0.58);
- higher levels of deaths (20% vs 11%); that is, a 38% relative increase (aRR=1.38, 95% CI: 1.00 to 1.91);
- higher levels of loss to follow-up (15% vs 12%); that is, a 33% relative increase (aRR=1.33, 95% CI: 0.97 to 1.81);
- similar levels of AEs (5.0% vs 4.7%), (aRR=1.00, 95% CI: 0.59 to 1.69); and
- lower levels of amplification of drug resistance (0.6% vs 1.4%); that is, a 73% relative reduction (aRR=0.27, 95% CI: 0.12 to 0.61).
The GDG judged both the benefits of the 9-month regimen with linezolid and the undesirable effects to be moderate compared with the longer regimens. The certainty of evidence was judged to be very low. Based on this, the GDG judged that the balance of health effects did not favour either the 9-month regimen with linezolid or the longer regimens. The panel judged that although the balance of effects did not favour either the intervention or the comparator, several other criteria in the GRADE evidenceto-decision tables (e.g. resources, acceptability, equity and feasibility) favoured the 9-month regimen.
Conclusion
The use of either the 9-month regimen with linezolid or the longer (18-month) regimens is suggested in people with pulmonary MDR/RR-TB without fluoroquinolone resistance (conditional recommendation, very low certainty of evidence).
Summary of other evidence
During assessment of sub-PICO 1.1, the panel noted that the cost of component medicines is likely to be similar because both regimens are of the same duration and use the same component medicines except for one – linezolid instead of ethionamide. The duration of linezolid use is 2 months compared with 4 months for ethionamide. Based on GDF prices (50) the cost difference was negligible (2 months of linezolid at 600 mg/day US$ 21, and 4 months of ethionamide at 450 mg/day US$ 32).
The health care costs are also likely to be similar because the two regimens are of the same duration and have the same component medicines, except for one – linezolid instead of ethionamide.
The panel also assumed no difference in DST needs. Both regimens are indicated for patients with MDR/RR-TB and without fluoroquinolone resistance. These patients are usually tested for rifampicin and fluoroquinolone resistance – rapid DSTs for both of these medicines are available. It might also be useful to perform genotypic DST because mutations in the inhA gene also confer resistance to ethionamide.
Evidence to recommendations: considerations
In 2022, new evidence from programmatic implementation in South Africa was made available to WHO where the regimen was modified to include 2 months of linezolid (600 mg) instead of 4 months of ethionamide.
Based on an assessment of the certainty of the evidence, carried out using predefined criteria and documented in the GRADEpro software, the certainty of the evidence was rated as very low for both comparisons.
Table 2.1 lists the comparisons and decisions on each of the sub-PICO questions that were assessed by the GDG to conclude with the summary recommendation (Recommendation 2.1). The main assessment that defined the overall decision was based on sub-PICO 1.1. The background for this decision was provided by the previous review and recommendation for the use of the 9-month regimen with ethionamide agreed during the GDG meeting in November 2019 and reflected in the recommendations published in the 2020 DR-TB treatment guidelines update (29).
Table 2.1. PICO questions and decisions of the GDG panel
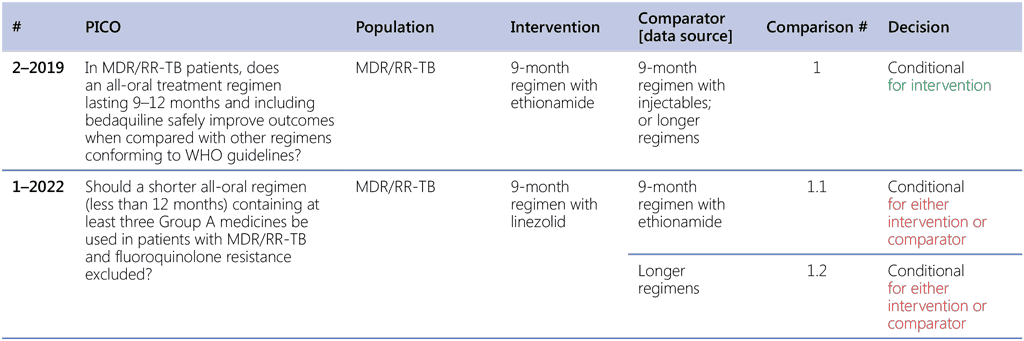
GDG: Guideline Development Group; MDR/RR-TB: multidrug-resistant or rifampicin-resistant TB; PICO: population, intervention, comparator and outcome; TB: tuberculosis; WHO: World Health Organization.
Sub-PICO 1.1
The GDG acknowledged that, during the analysis, the intervention and comparator groups were made as comparable as possible. However, the GDG considered possible unmeasured confounding due to a lack of systematic collection of information on comorbidities and radiological findings through the EDRWeb system, as well as methodological challenges (e.g. a potential selection bias). Apart from the selection criteria listed, the risk of major selection bias was considered to be low, given that this intervention represented a complete and comprehensive switch in the countrywide programmatic approach.
Regarding generalizability, the GDG deliberated whether the genetic diversity of M. tuberculosis strains in South Africa was comparable to strains present in other settings; the group concluded that strains found in other settings were adequately represented in the country. The group also considered potential interactions in relation to HIV status and the effect of ART, but this was not considered a major factor given that treatment outcomes were similar in PLHIV and HIV-negative people. The GDG agreed that results of the STREAM Stage 2 trial – a large-scale, multicountry Phase 3 trial examining a shorter all-oral bedaquiline-containing regimen – will provide additional important insight into the efficacy and safety of this regimen, and may increase the certainty of the evidence.
A clear limitation emphasized by the GDG was the lack of data on AEs in the EDRWeb. No direct comparative evidence was available on AEs because the data on such events were not systematically collected for the 9-month regimen with ethionamide. The rate of Grade 3–5 AEs was 5% for the 9-month regimen with linezolid. The panel nevertheless considered the potential AEs of both ethionamide and linezolid in balancing the benefits and harms (Table 2.2).
Table 2.2. Summary of AEs associated with linezolid and ethionamide

The panel also considered the duration and pill burden with the intervention and comparator regimens. Both regimens have the same duration, so neither offers an advantage of shorter treatment, although the duration of the linezolid regimen is shorter than that of ethionamide. The pill burden is likely to be slightly lower with the intervention because linezolid is prescribed for 2 months in the 9-month regimen with linezolid and ethionamide for 4 months in the 9-month regimen with ethionamide.
Considering this evidence, the panel judged that the 9-month regimen with linezolid may have small desirable effects and noted the very low certainty of the evidence. Certainty of the evidence was rated “very low” for all outcomes on account of potential misclassification bias and confounding bias (downgraded 1 level), and serious indirectness (downgraded 1 level). The overall certainty is generally based on the lowest certainty for the agreed critical outcomes; thus, it was judged to be very low. The panel noted that the evidence on both the intervention and on the comparator regimen was obtained from programmatic data from South Africa such that, overall, the population and health care context were comparable. However, the panel stressed that important differences exist between the two cohorts or datasets that were compared, making it difficult to draw conclusions with full confidence.
The panel judged that there was probably no important uncertainty or variability in how much people value the main outcomes. The panel used available data on cost of component medicines combined with professional judgement to estimate the cost of the 9-month regimen with linezolid compared with the 9-month regimen with ethionamide among patients with MDR/RR-TB, susceptible to fluoroquinolones. The panel suggested that the cost would be expected to be very similar; that is, for there to be negligible costs or savings. The panel also noted that no data were available on the cost of managing potential long-term consequences of neurotoxicity that can be caused by the use of linezolid, and that the risk is greater if linezolid is used for longer periods. The panel has also noted that health care and patient costs are likely to be similar for regimens when used in a similar group of patients and for the same duration.
The GDG attempted to discuss cost–effectiveness of the two regimens; however, no evidence was available, the two regimens are identical in duration and they only differ in one component drug, which would not change the overall cost of the regimen in any significant way. The similarity of the two regimens also prevented a substantial discussion on the equity. The panel considered patients and health care providers as key stakeholders. The panel considered the following aspects as critical with regard to acceptability: regimen duration, drug-safety monitoring needs (relating both to the necessary travel, loss of income and general disruption of the life of patients, and to workload for the health care system) and DST needs. The panel judged that there were probably no differences in acceptability between the 9-month regimen with linezolid and the 9-month regimen with ethionamide, given the overall similarity of the regimens, and that the 9-month regimen with linezolid would probably be acceptable. The panel considered the following aspects to affect feasibility (i.e. to be potential barriers to implementation): requirements for drug-safety monitoring and for DST. The 9-month regimen with linezolid would require monitoring of toxicity (e.g. anaemia) and DST.
The panel judged that the balance of desirable and undesirable consequences favours neither the 9-month regimen with linezolid nor the 9-month regimen with ethionamide in this population. Specifically, the panel felt that there is a fine balance between the two options in terms of benefits and harms that is uncertain given the overall very low certainty in the evidence (due to potential misclassification bias, confounding bias and serious indirectness). The panel judged that for most other evidence-to-decision criteria (e.g. resources, acceptability and feasibility) there was unlikely to be a large difference between the 9-month regimen with linezolid and the 9-month regimen with ethionamide because the only difference between the two regimens is the replacement of ethionamide with linezolid. Overall, the panel judged that either regimen could be used and that the flexibility of using either linezolid or ethionamide was helpful to optimize patient care. These considerations also guided the agreement of the panel on the strength of the recommendation being conditional.
Sub-PICO 1.2
The GDG acknowledged that, during the analysis, the intervention and comparator groups were made as comparable as possible. The panel noted that the evidence on the 9-month regimen was obtained from programmatic data from South Africa, whereas the evidence on the longer regimen represented only subsets of patients from the countries and researchers that submitted data. The panel also noted substantial inconsistency between cohorts in the comparator group (on the longer regimens). Overall, there was concern that the selective nature of the data on the longer regimens may have biased the comparison in favour of the longer regimen. As a result, there were serious concerns about the comparability of the data, making it difficult to draw conclusions with confidence. The panel also considered the duration and overall pill burden with the intervention and comparator regimens, which are both lower in the 9-month regimen and thus represent a benefit of the intervention.
Considering this evidence and the totality of observed effects of the 9-month regimen with linezolid on the outcomes, the panel judged that the 9-month regimen with linezolid may have moderate desirable effects and that it may also have moderate undesirable effects.
Certainty in the estimates was rated “very low” for all outcomes owing to very serious risk of bias (potential misclassification bias and confounding bias), inconsistency (inconsistency in the effect estimates among 14 comparator cohorts) and indirectness (with data for the intervention regimen being from a single country). The overall certainty is generally based on the lowest certainty for the agreed critical outcomes and thus was judged to be very low.
The panel noted that the costs for people with MDR/RR-TB receiving the 9-month regimen with linezolid are expected to be lower than those for longer regimens (18 months or longer) because costs for drugs, care and monitoring are expected to be lower.
The panel considered the ability to decentralize treatment (to enable access for remote, underserviced settings and disadvantaged populations) as affecting equity. Despite not being able to identify relevant research evidence, the panel used their collective experience to judge that there would probably be advantages associated with the use of the 9-month regimen owing to its reduced complexity and shorter duration. The panel judged that use of the 9-month regimen with linezolid would probably increase equity.
The panel considered patients and health care providers as key stakeholders and the following aspects as critical with regard to acceptability: regimen duration and drug safety, monitoring needs (relating both to the necessary travel, loss of income and general disruption of the life of patients, and to workload for the health care system) and needs for DST. The panel judged that the 9-month regimen with linezolid would probably be acceptable to key stakeholders.
The balance of desirable and undesirable consequences was judged to not favour either the use of the 9-month regimen or the longer, 18-month regimens in this population. Specifically, the panel felt that there is a fine balance between the two options in terms of benefits and harms that is uncertain given the overall very low certainty in the evidence. The panel judged that although the balance of effects did not favour either the intervention or the comparator, several other evidence-to-decision table criteria (e.g. resources, acceptability, equity and feasibility) favoured the 9-month regimen.
Overall, the panel judged that either regimen could be used in the eligible patient group presented in the analysis; they noted the more limited eligibility for the 9-month regimen and acknowledged that the applicability of the longer, individualized regimens is more flexible and significantly broader, including many patient groups that are not eligible for the shorter regimen. These considerations have also guided the agreement of the panel on the conditionality of this recommendation.
Subgroup considerations
Based on research evidence and expert experience, the panel identified subpopulations of people who might be affected differently than most by this recommendation; these subpopulations were PLHIV, children, pregnant women, breastfeeding women, patients with extrapulmonary TB and patients with extensive TB disease. The recent new recommendation for use of bedaquiline in children with MDR/RR-TB aged below 6 years was considered (30). The panel noted specific considerations for the subpopulations listed below.
People living with HIV
The data evaluated corresponded to a setting with a high prevalence of HIV; of particular significance was that most PLHIV (>90%) who started the 9-month regimens were receiving ART. In view of the treatment outcomes described in the analysis, there were no grounds to believe that the regimen would perform any differently in PLHIV. It is necessary to consider significant clinical interactions that may increase bedaquiline exposure or that of other agents with potential for cardiotoxicity when these are co-administered with antiretroviral drugs. However, because the data evaluated did not include information on changes to the regimen as a result of management of adverse drug reactions, or complications from drug–drug interactions, the GDG reiterated that it is worth paying attention to any potential drug–drug interactions or overlapping drug toxicities that may not have been captured. For example, bedaquiline concentrations can be reduced by efavirenz (these drugs should not be co-administered) or increased by boosted protease inhibitors (resulting in a need for greater vigilance in monitoring for drug-related QT effects) (56–58). Neuropathy, liver enzyme elevations and CNS sideeffects can be attributed to HIV or TB drugs or their interactions (59).
Children and adolescents
The datasets included only small numbers of people aged below 15 years (n=69), and thus did not allow for reliable comparisons in both datasets from South Africa (n=69 and n=7) and in the 2021 IPD (n=7). However, analysis in the subgroup aged below 15 years showed a relative increase in treatment success of 42% (aRR=1.42, 95% CI: 0.7 to 2.89) in sub-PICO 1.1 and a 5% relative reduction (RR=0.95, 95% CI: 0.78 to 1.15) in sub-PICO 1.2. Although a small number of participants were aged between 10 and 15 years (19/50, 38% in the intervention group, and 75/162, 46% in the comparator group), extrapolation of the findings to children was deemed reasonable for efficacy because components of the regimen had been used safely in children based on other available data regarding linezolid use in children. This extrapolation was considered applicable to children of all ages, taking into account the recommendation for use of bedaquiline in children aged below 6 years (30).
Pregnant and breastfeeding women
In the research studies analysed, pregnant women were not identified, and subgroup data were unavailable. Ethionamide is usually contraindicated in pregnancy (because animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans), and this is the main reason that the 9-month regimen has not been recommended for this subgroup in the past. There is experience in using linezolid during pregnancy (60, 61). For pregnant and breastfeeding women, it is therefore recommended to use the regimen with linezolid instead of ethionamide.
Extrapulmonary TB
A subgroup of people with extrapulmonary TB were included in the research studies (81 in the regimen containing linezolid and 23 in the regimen with ethionamide). In view of the unavailability of evidence on surrogates for severity or extent of disease, the use of this regimen in patients with severe forms of extrapulmonary TB is not recommended.
Implementation considerations
Drug susceptibility testing
DST for bedaquiline and linezolid is an important implementation consideration that will need to be enhanced in many countries, given the increasing use of these medicines in all regimens for MDR/RR-TB and the possible further inclusion of new medicines in MDR-TB treatment regimens. The implementation of these recommendations must be accompanied by continued efforts to increase access to DST for all medicines for which reliable methods are currently available, and for the development and roll-out of DST methods for newer medicines.
Access to WHO-recommended rapid DST is essential, especially for detecting resistance to rifampicin and fluoroquinolones, before starting the 9-month regimens. Baseline DST will confirm eligibility for different regimen options; therefore, the establishment and strengthening of DST services is a vital consideration for implementation. The DST methods for identifying resistance to bedaquiline and linezolid have been developed on available phenotypic platforms and need to be implemented in all settings where these medicines are being used. Resistance to other anti-TB drugs should be monitored in accordance with WHO recommendations.
One of the exclusion criteria for all shorter regimens in the datasets from South Africa was mutations in both inhA promoter and katG regions, confirmed using a line probe assay (LPA). This means that patients with only inhA or only katG mutations were included. A first-line LPA (MTBDRplus) and Xpert MTB/XDR cartridge can determine mutations in the inhA promoter or katG regions; both mutations confer resistance to isoniazid, with the resistance being low level when inhA mutations alone are present, or high level with katG gene mutations alone or inhA promoter and katG gene mutations combined. Mutations at the inhA promoter are also associated with resistance to ethionamide and prothionamide. The presence of mutations in both the inhA promoter and katG suggests that isoniazid at high dose and thioamides are not effective, and that the 9-month regimen may not therefore be used. In the absence of information on mutation patterns for an individual patient, the decision can be informed by knowledge of the frequency of the concurrent occurrence of both mutations, obtained from drug-resistance surveillance (62). Phenotypic DST for some medicines included in the regimen (e.g. ethambutol and ethionamide) is not considered reliable and reproducible; therefore, this testing should be employed with caution to inform the use of this regimen.³⁶
Currently, there is limited capacity globally to carry out DST for bedaquiline; however, laboratory capacity should be strengthened in this area as new medicines and regimens begin to be used more widely. National and reference laboratories will need to have the relevant reagents available to enable DST to be carried out and will need data on the MIC distribution of all M. tuberculosis lineages that are circulating globally. The WHO TB SRL Network is available to support national TB reference laboratories in performing quality-assured DST. A WHO technical consultation in 2017 established critical concentrations for susceptibility testing for the fluoroquinolones, bedaquiline, delamanid, clofazimine and linezolid (43).
Selection of fluoroquinolones
Selection of fluoroquinolones may take into account the evidence from South Africa available for the review – 83% of patients analysed using the 2017 dataset received levofloxacin and the rest received moxifloxacin at standard dose (400 mg daily). Both levofloxacin and moxifloxacin have shown similar efficacy for treating DR-TB. The choice between levofloxacin and moxifloxacin was guided by the potential risk of cumulative cardiotoxicity, using moxifloxacin in a shorter regimen with injectables and levofloxacin in an all-oral shorter regimen. Levofloxacin is often preferred because of moxifloxacin’s slightly higher potential for cardiotoxicity; however, levofloxacin has been associated with musculoskeletal disorders in paediatric populations. Therefore, irrespective of the choice of fluoroquinolone, NTPs need to implement aDSM in all patients enrolled on treatment of DR-TB (64, 65).
Assessment of TB disease
To determine regimen options, it is important to know the extent of TB disease, in addition to the DST results and other considerations mentioned above. Extensive TB disease is defined in this document as the presence of bilateral cavitary disease or extensive parenchymal damage on CXR. In children aged below 15 years, advanced disease is usually defined by the presence of cavities or bilateral disease on CXR. This highlights the importance of CXR as part of the diagnostic and clinical management work-up for patients.
Regimen duration
The regimen comprises an intensive phase of 4 months that may be extended to 6 months when no bacteriological conversion is seen at the end of the fourth month of treatment, and a continuation phase of 5 months; hence, if extended, the regimens may last 11 months. In the dataset reviewed, the duration of bedaquiline and linezolid was restricted to 6 and 2 months, respectively.
Patient-centred approach
Efforts are required to provide patient support to enable full adherence to treatment.
Recommendations 2.2 and 2.3 The modified 9-month all-oral regimens for MDR/RR-TB
Remarks
- The recommended modified 9-month all-oral regimens comprise bedaquiline, linezolid and pyrazinamide in different combinations with levofloxacin/moxifloxacin, clofazimine and delamanid.
- This recommendation applies to the following:
- People with MDR/RR-TB and in whom resistance to fluoroquinolones has been excluded.
- People with diagnosed pulmonary TB, including children, adolescents, PLHIV, and pregnant and breastfeeding women.
- People with extensive TB disease and all forms of extrapulmonary TB except for TB involving the CNS, osteoarticular TB or disseminated forms of TB with multiorgan involvement.
- People with MDR/RR-TB and less than 1 month of previous exposure to any of the component medicines of the regimen (apart from pyrazinamide and fluoroquinolones). When exposure is greater than 1 month, these patients may still receive one of the regimens if resistance to the specific medicines with such exposure has been ruled out.
- Children and adolescents who do not have bacteriological confirmation of TB or resistance patterns but do have a high likelihood of MDR/RR-TB (based on clinical signs and symptoms of TB, in combination with a history of contact with a patient with confirmed MDR/RR-TB).
Rationale
The rationale for these recommendations is based on the evidence and considerations detailed in the subsections below. These 9-month regimens (BLMZ, BLLfxCZ and BDLLfxZ) can be used in patients with MDR/RR-TB (in whom resistance to FQ has been excluded) who cannot be offered one of the 6-month regimens. These regimens are the preferred option over the longer (>18-month) regimens, and the GDG panel has advised a relative ranking of preference among the three modified 9-month regimens based on the review of multiple criteria.
The evidence from the endTB trial (NCT02754765) (66) of all five regimens was assessed. The outcomes of participants in five trial arms receiving modified 9-month regimens were compared with the trial’s comparator arm, in which most participants received longer regimens. All five experimental regimens were of 9 months duration. Three of these regimens included bedaquiline (for the whole duration of the regimen), a quinolone (either moxifloxacin or levofloxacin), linezolid, pyrazinamide, and either delamanid or clofazimine, or no fifth drug. The two remaining regimens were without bedaquiline but included delamanid, a quinolone (moxifloxacin or levofloxacin), clofazimine and pyrazinamide, with or without linezolid as a fifth medicine (see Table 2.4). The assessment of three bedaquiline-containing regimens showed higher levels of treatment success (89%, 88.7% and 85.2% vs 77%), slightly higher or lower failure or recurrence (3.4%, 6.1% and 1.6% vs 2.5%), lower levels of deaths (1.7%, 0.9% and 2.5% vs 3.4%), lower levels of LTFU (5.9%, 4.3% and 10.7% vs 16.8%) and similar levels of amplification of drug resistance (0.0%, 1.6% and 0.0% vs 0%) than the comparator. The two regimens without bedaquiline showed variable levels of treatment success (76.3% and 84.6% vs 77%), failure or recurrence (11% and 8.7% vs 2.5%), deaths (2.5% and 2.9% vs 3.4%) and LTFU (10.2% and 3.8% vs 16.8%), and higher amplification of drug resistance (4.0% and 6.7% vs 0%).
Based on a review of these five assessments, it was considered that three bedaquiline-containing regimens (BLMZ, BLLfxCZ and BDLLfxZ) can be suggested for use in preference to currently recommended longer (>18 months) regimens in patients with MDR/RR-TB and in whom resistance to FQ has been excluded. Additional assessment of multiple factors (including resource requirements, health equity, acceptability and access to delamanid) led to the conclusion that BLMZ is preferred over BLLfxCZ, and that BLLfxCZ is preferred over BDLLfxZ, because the net health effects appeared to be most favourable for BLMZ, followed by BLLfxCZ. The endTB trial enrolled patients with important comorbidities and patients with extensive TB disease; therefore, in contrast to the recommendation for the 9-month all-oral regimen, this recommendation can be extended to these groups of patients.
Summary of evidence
This section provides the PICO questions posed, the data and studies considered to answer the questions, the methods used for analysis and data synthesis, and summaries of evidence on desirable and undesirable effects, certainty of evidence and other evidence considered during the recommendation’s development. Additional detail on the evidence is available in the annexes containing the GRADE evidence summary tables and GRADE evidence-to-decision tables (Annex 5).
PICO questions
The recommendations in this section result from assessments of the PICO questions listed below.
PICO question 2–2024 (MDR/RR-TB, 2024): Should any 9-month endTB trial regimens be used in patients with pulmonary RR-TB (without FQ resistance) over the currently recommended longer regimens?
Because of the different interventions tested, PICO 2 has been split into several sub-PICO questions, as shown in Table 2.3.
PICO question 2.1–2024 (MDR/RR-TB, 2024): Should a 9-month regimen using bedaquiline, linezolid, moxifloxacin, and pyrazinamide (9BLMZ) vs. currently recommended longer WHO regimens be used in patients with pulmonary RR-TB (without fluoroquinolone resistance)?
PICO question 2.2–2024 (MDR/RR-TB, 2024): Should a 9-month regimen using bedaquiline, clofazimine, linezolid, levofloxacin, and pyrazinamide (9BLLfxCZ) vs. currently recommended longer WHO regimens be used in patients with pulmonary RR-TB (without fluoroquinolone resistance)?
PICO question 2.3–2024 (MDR/RR-TB, 2024): Should a 9-month regimen using bedaquiline, delamanid, linezolid, levofloxacin, and pyrazinamide (9BDLLfxZ) vs. currently recommended longer WHO regimens be used in patients with pulmonary RR-TB (without fluoroquinolone resistance)?
PICO question 2.4–2024 (MDR/RR-TB, 2024) Should a 9-month regimen using delamanid, clofazimine, linezolid, levofloxacin, and pyrazinamide (9DCLLfxZ) vs. currently recommended longer WHO regimens be used in patients with pulmonary RR-TB (without fluoroquinolone resistance)?
PICO question 2.5–2024 (MDR/RR-TB, 2024) Should a 9-month regimen using delamanid, clofazimine, moxifloxacin, and pyrazinamide (9DCMZ) vs. currently recommended longer WHO regimens be used in patients with pulmonary RR-TB (without fluoroquinolone resistance)?
Table 2.3. Sub-PICO questions to PICO 2
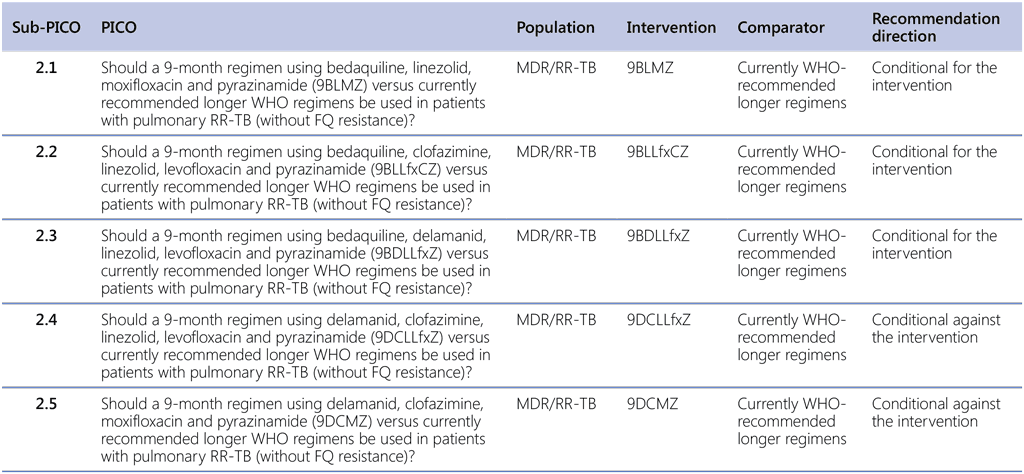
FQ: fluoroquinolone; MDR/RR-TB: multidrug-resistant or rifampicin-resistant TB; PICO: population, intervention, comparator and outcome; RR-TB: rifampicin-resistant TB; TB: tuberculosis; WHO: World Health Organization.
Data and studies considered
This group of PICO questions was reviewed during the GDG meeting convened by WHO in June 2024, using the evidence from the endTB trial (66). The endTB trial was an RCT that was led by Partners In Health (PIH), Médecins Sans Frontières (MSF), and Interactive Research and Development (IRD); the aim was to improve the efficacy and safety of treatment for patients with FQ-susceptible MDR/RR-TB.
endTB trial
The endTB trial was a Bayesian response-adaptive randomized Phase 3, multicountry, controlled, parallel, open-label clinical trial. Participants were randomly assigned to either the control arm, which follows the SoC longer (18-month) regimens for MDR/RR-TB, or to one of five 39-week multidrug regimens that incorporate newly approved and repurposed drugs. The duration of follow-up for all arms ranged from a minimum of 73 weeks to a maximum of 104 weeks post-randomization.
The primary objective of the endTB trial was to assess whether the efficacy of each experimental regimen is non-inferior to that of the control. The endTB clinical trial started in 2017 and randomized and followed 754 participants over 7 years and across 12 sites in seven countries: Georgia, India, Kazakhstan, Lesotho, Pakistan, Peru and South Africa.
Eligible participants were aged at least 15 years and had pulmonary TB suspected or confirmed to be resistant to rifampicin and susceptible to FQ. Participants living with HIV (regardless of CD4 count), diabetes (regardless of A1c), substance use disorders and various degrees of TB disease severity were enrolled in the trial. All study arms were similar in size (ranging from 107 to 122) with 699 participants in the modified intention-to-treat (mITT) population for the analysis.
All five experimental regimens were of 9 months (i.e. 39 weeks) duration. Three of these regimens included bedaquiline for the whole duration of the regimen (400 mg once daily for the first 2 weeks of treatment, followed by 200 mg 3 times per week), a quinolone (either moxifloxacin or levofloxacin), linezolid, pyrazinamide, and either delamanid (100 mg BID) or clofazimine or no fifth drug. The remaining two regimens were without bedaquiline but included delamanid, a quinolone (moxifloxacin or levofloxacin), clofazimine and pyrazinamide, with or without linezolid as a fifth medicine (see Table 2.4). All patients who received linezolid started at 600 mg daily but were later randomized to a reduced dose (300 mg daily or 600 mg 3 times a week) at 16 weeks or earlier in case of doselimiting toxicity.
Table 2.4. endTB trial regimens
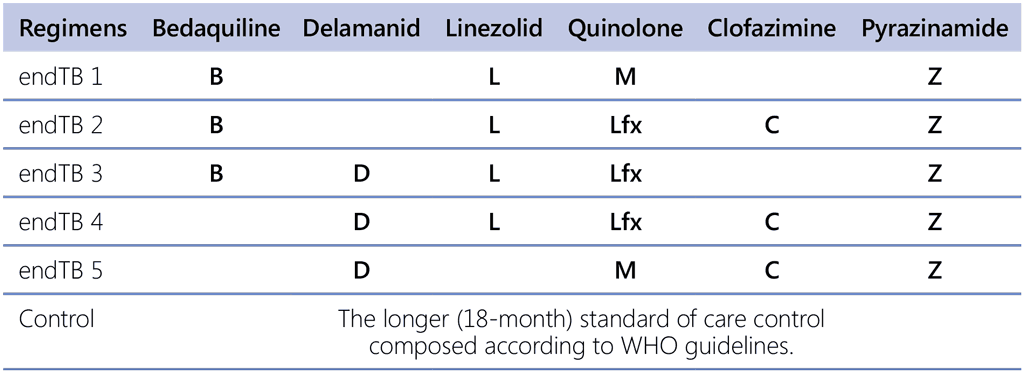
Lfx: levofloxacin; M: moxifloxacin; WHO: World Health Organization.
Methods used for analysis and data synthesis
Descriptive analyses
Descriptive analyses of the baseline characteristics of participants in the study were performed. Characteristics included demographics, pregnancy status and laboratory parameters such as HIV status, CD4 count (if applicable), drug susceptibility tests and diagnostic test results, TB treatment received before randomization, AEs and treatment regimens, and end-of-treatment and end-of-follow-up outcomes.
Statistical analyses
Statistical analyses were based on the WHO outcome definitions listed in Annex 2.
The endTB trial specified follow-up for at least 73 weeks, but most participants were followed up until the end of the control regimen and 104 weeks post-randomization. The outcome was assigned for endpoints at 73 and 104 weeks. For the WHO review, the efficacy analyses used the week 104 endpoint. The study team performed the analyses and presented them to the WHO panel.
The endTB outcome definitions were similar to the WHO outcome definitions, except for the LTFU. Patients who were originally classified as being LTFU based on the endTB protocol and statistical analysis plans (and thus assigned an unfavourable outcome) were reclassified as “sustained treatment success” if all of the following conditions were met:
- the participant had completed treatment;
- the participant had been assigned an unfavourable outcome at week 104 based on the endTB protocol solely because of missed visits, LTFU, or withdrawal of consent; and
- the participant had at least one negative culture and no positive cultures after treatment completion.
Table 2.5. Cross-tabulation of endTB and WHO outcomes at week 104 – mITT population
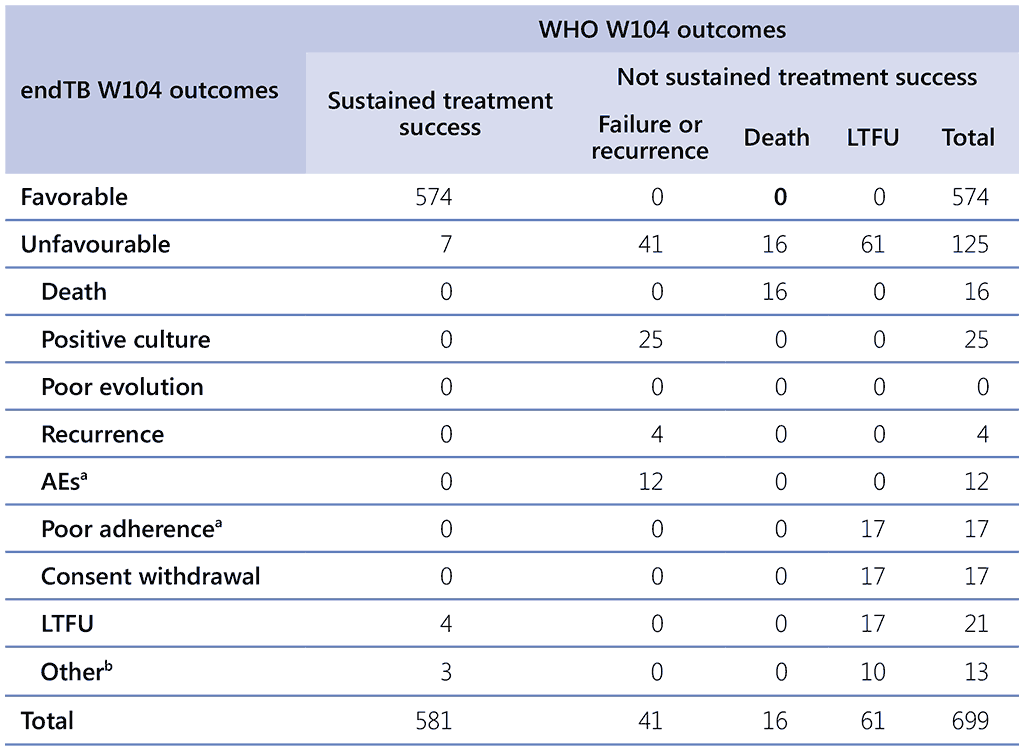
AE: adverse event; LTFU: loss to follow-up; mITT: modified intention-to-treat; WHO: World Health Organization.
a Treatment discontinued for specified reasons
b Not assessable after completing treatment (6), Investigator’s judgement (4), Pregnancy or breastfeeding (2), Use of prohibited concomitant medication (1)
The evidence on the novel regimens to inform PICO questions was derived from one trial. Data from patients in relevant arms of this trial were used in each of the endTB trial comparisons that led to the conclusions and final recommendation on the use of the BLMZ, BLLfxCZ and BDLLfxZ.
Table 2.6. High-level summary of main inclusion and exclusion criteria: endTB trial

Decision thresholds
In contrast to previous recommendations, a new method of determining the magnitude of the health effects was used. A triangulation approach was used to develop outcome-specific decision thresholds (DTs) for judging the magnitude of the effects for the following health outcomes: death, sustained treatment success, treatment failure or recurrence, LTFU, AEs and amplification of drug resistance. These outcomes were deemed critical or important for decision-making based on a prioritization survey of the GDG members using the GRADE approach. The survey included health outcome descriptors that had previously been developed for each of the health outcomes to facilitate understanding of the outcomes by the GDG during their decision-making process.
The GDG first reviewed judgements about the magnitude of health effects made by other GDGs for previous WHO MDR-TB guidelines (3, 14) to determine approximate ranges of effect sizes that the group considered to be trivial, small, moderate or large. Members then identified a systematic review to help inform suggested health utility values for the health state of having DR-TB disease and treatment success (about 0.5) and treatment success (about 0.9) (46). For the other outcomes (treatment failure, LTFU, amplification of drug resistance and AEs), a health utility value of 0.5 was used, considering that these would be similar health states to having DR-TB disease, and to align with previous judgements made in other TB guidelines.
Second, the group used the empirical evidence from the GRADE THRESHOLD trial (47) to calculate suggested utility-adjusted absolute effect thresholds for the health outcomes. The calculated thresholds were as follows (48):
- death (health utility: 0):
- trivial or no effect: ≤14 fewer or more deaths per 1000 people;
- small effect: 15–32 fewer or more deaths per 1000 people;
- moderate effect: 33–63 fewer or more deaths per 1000 people;
- large effect: ≥64 fewer or more deaths per 1000 people;
- sustained treatment success (health utility: 0.9):
- trivial or no effect: ≤15 fewer or more treatment successes per 1000 people;
- small effect: 16–35 fewer or more treatment successes per 1000 people;
- moderate effect: 36–68 fewer or more treatment successes per 1000 people;
- large effect: ≥69 fewer or more treatment successes per 1000 people;
- treatment failure or recurrence, LTFU, AEs and amplification (acquisition) of drug resistance (all with health utility 0.5):
- trivial or no effect: ≤30 fewer or more failures or recurrences per 1000 people;
- small effect: 31–59 fewer or more failures or recurrences per 1000 people;
- moderate effect: 60–119 fewer or more failures or recurrences per 1000 people; and
- large effect: ≥120 fewer or more failures or recurrences per 1000 people.
These suggested thresholds (assumed to occur over the duration of follow-up in the trials) were generally consistent with judgements that were made in the previous WHO MDR-TB guidelines (3).
Third, in preparation for the GDG meeting at which recommendations would be formulated, an online survey was administered to the group to obtain their feedback on the suggested DTs. The survey asked members to agree with the suggested thresholds, or to disagree and suggest alternative thresholds based on their expert experience. The agreed-upon thresholds were again reviewed at the start of the GDG meeting, and the group decided to use those thresholds to inform their judgements about the magnitude of health effects in the GRADE EtD frameworks. Figures were created to visually depict absolute effects and 95% CIs from the research evidence of the relevant trial in relation to the DTs for each health outcome, to facilitate the GDG’s discussion and judgements about whether the health effects were trivial, small, moderate or large, and to judge the level of imprecision of estimates (Fig. A1.3). The same thresholds were used to inform the group’s judgements about imprecision, in line with GRADE guidance for decision-making (49).
Summary of evidence on desirable and undesirable effects and certainty of evidence
Sub-PICO 2.1
Patients with MDR/RR-TB receiving the BLMZ regimen (n=118 for death, failure and recurrence and LTFU; n=126 for AEs and n=127 for amplification of drug resistance) compared with those receiving the currently recommended longer WHO regimens (n=119 for death, failure and recurrence and LTFU; n=126 for AEs and n=130 for amplification of drug resistance) experienced:
- lower levels of death: 1.7% versus 3.4%; RD=17 fewer per 1000 (95% CI: from 57 fewer to 23 more per 1000);
- lower levels of LTFU: 5.9% versus 16.8%; RD=109 fewer per 1000 (95% CI: from 188 fewer to 29 fewer per 1000);
- lower levels of people with at least one grade 3 to 5 AEs: 55.6% versus 65.1%; RD=95 fewer per 1000 (95% CI: from 216 fewer to 25 more per 1000);
- lower levels of people with at least one serious AE: 15.9% versus 19.0%; RD=32 fewer per 1000 (95% CI: from 125 fewer to 62 more per 1000);
- similar levels of amplified resistance: 0.0% versus 0.0%; RD=0 fewer per 1000 (95% CI: from 29 fewer to 29 more per 1000); and
- higher levels of failure or recurrence: 3.4% versus 2.5%; RD=9 more per 1000 (95% CI: from 34 fewer to 52 more per 1000).
The GDG judged the benefits of BLMZ to be moderate and the undesirable effects to be trivial compared with WHO recommended longer regimens. The certainty of evidence was judged to be overall very low, with probably no important uncertainty in the values that people place on the outcomes. Hence, the GDG determined that the balance of health effects probably favours the BLMZ regimen.
Conclusion
The BLMZ regimen (composed of bedaquiline, linezolid, moxifloxacin and pyrazinamide) is suggested over currently recommended longer regimens in patients with FQ-susceptible RR-TB.
Sub-PICO 2.2
Patients with MDR/RR-TB receiving the BLLfxCZ regimen (n=115 for death, failure and recurrence and LTFU and n=122 for AEs; and n=124 for amplification of drug resistance) compared with those receiving the currently recommended longer WHO regimens (n=119 for death, failure and recurrence and LTFU and n=126 for AEs; and n=130 for amplification of drug resistance) experienced:
- lower levels of death: 0.9% versus 3.4%; RD=25 fewer per 1000 (95% CI: from 62 fewer to 12 more per 1000);
- lower levels of LTFU: 4.3% versus 16.8%; RD=125 fewer per 1000 (95% CI: from 201 fewer to 48 fewer per 1000);
- lower levels of people with at least one Grade 3 to 5 AEs: 59.0% versus 65.1%; RD=61 fewer per 1000 (95% CI: from 181 fewer to 60 more per 1000);
- lower levels of people with at least one serious AE: 14.8% versus 19.0%; RD=43 fewer per 1000 (95% CI: from 136 fewer to 50 more per 1000);
- higher levels of failure or recurrence: 6.1% versus 2.5%; RD=36 more per 1000 (95% CI: from 16 fewer to 123 more per 1000); and
- higher levels of amplified resistance: 1.6% versus 0%; RD=16 more per 1000 (95% CI: from 13 fewer to 56 more per 1000).
The GDG judged the benefits of BLLfxCZ to be moderate and the undesirable effects to be small compared with WHO-recommended longer regimens. The certainty of evidence was judged to be very low overall, with probably no important uncertainty in the values that people place on the outcomes. Hence, the GDG determined that the balance of health effects probably favours the BLLfxCZ regimen.
Conclusion
The BLLfxCZ regimen composed of bedaquiline, linezolid, levofloxacin, clofazimine and pyrazinamide is suggested over currently recommended longer regimens in patients with FQ-susceptible RR-TB.
Sub-PICO 2.3
Patients with MDR/RR-TB receiving the BDLLfxZ regimen (n=122 for failure and recurrence, death, and LTFU; n=127 for AEs and n=128 for amplification of drug resistance) compared with those receiving the currently recommended longer WHO regimens (n=119 for failure and recurrence, death and LTFU; n=126 for AEs and n=130 for amplification of drug resistance) experienced:
- lower levels of failure or recurrence: 1.6% versus 2.5%; RD=9 fewer per 1000 (95% CI: from 45 fewer to 27 more per 1000);
- lower levels of death: 2.5% versus 3.4%; RD=9 fewer per 1000 (95% CI: from 52 fewer to 33 more per 1000);
- lower levels of LTFU: 10.7% versus 16.8%; RD=62 fewer per 1000 (95% CI: from 148 fewer to 25 more per 1000);
- lower levels of people with at least one Grade 3 to 5 AEs: 63.0% versus 65.1%; RD=21 fewer per 1000 (95% CI: from 139 fewer to 97 more per 1000);
- lower levels of people with at least one serious AE: 15.7% versus 19.0%; RD=33 fewer per 1000 (95% CI: from 126 fewer to 60 more per 1000); and
- similar levels of amplified resistance: 0.0% versus 0.0%; RD=0 fewer per 1000 (95% CI: from 29 fewer to 29 more per 1000).
The GDG judged the benefits of BLLfxCZ to be small and the undesirable effects to be trivial compared with WHO-recommended longer regimens. The certainty of evidence was judged to be very low overall, with probably no important uncertainty in the values that people place on the outcomes. Hence, the GDG determined that the balance of health effects probably favours the BDLLfxZ regimen.
Conclusion
The BDLLfxZ regimen composed of bedaquiline, delamanid, linezolid, levofloxacin and pyrazinamide is suggested over currently recommended longer regimens in patients with FQ-susceptible RR-TB.
Sub-PICO 2.4
Patients with MDR/RR-TB receiving the DCLLfxZ regimen (n=118 for death, failure and recurrence and LTFU; n=124 for AEs and n=125 for amplification of drug-resistance) compared with those receiving the currently recommended longer WHO regimens (n=119 for death, failure and recurrence and LTFU and n=126 for AEs; and n=130 for amplification of drug resistance) experienced:
- lower levels of death: 2.5% versus 3.4%; RD=8 fewer per 1000 (95% CI: from 51 fewer to 35 more per 1000);
- lower levels of LTFU: 10.2% versus 16.8%; RD=66 fewer per 1000 (95% CI: from 153 fewer to 20 more per 1000);
- lower levels of Grade 3 to 5 AEs: 62.9% versus 65.1%; RD=22 fewer per 1000 (95% CI: from 141 fewer to 97 more per 1000);
- lower levels of people with at least one serious AE: 15.3% versus 19.0%; RD=37 fewer per 1000 (95% CI: from 131 fewer to 56 more per 1000);
- higher levels of failure or recurrence: 11.0% versus 2.5%; RD=85 more per 1000 (95% CI: from 22 more to 148 more per 1000); and
- higher levels of amplified resistance: 4.0% versus 0%; RD=40 more per 1000 (95% CI: from 9 more to 87 more per 1000).
The GDG judged the benefits of DCLLfxZ to be small and the undesirable effects to be moderate compared with WHO-recommended longer regimens. The certainty of evidence was judged to be very low overall, with probably no important uncertainty in the values that people place on the outcomes. Hence, the GDG determined that the balance of health effects probably favours the WHO-recommended longer regimens.
Conclusion
The GDG suggested against the use of the DCLLfxZ regimen in patients with FQ-susceptible RR-TB.
Sub-PICO 2.5
Patients with MDR/RR-TB receiving the DCMZ regimen (n=107 for death, failure and recurrence and LTFU; n=120 for AEs and for amplification of drug resistance) compared with those receiving the currently recommended longer WHO regimens (n=119 for death, failure and recurrence and LTFU and n=126 for AEs; and n=130 for amplification of drug resistance) experienced:
- lower levels of death: 2.8% versus 3.4%; RD=5 fewer per 1000 (95% CI: from 50 fewer to 41 more per 1000);
- lower levels of LTFU: 3.7% versus 16.8%; RD=131 fewer per 1000 (95% CI: from 207 fewer to 54 fewer per 1000);
- lower levels of people with at least one Grade 3 to 5 AEs: 60.0% versus 65.1%; RD=51 fewer per 1000 (95% CI: from 172 fewer to 70 more per 1000);
- lower levels of people with at least one serious AE: 17.5% versus 19.0%; RD=15 fewer per 1000 (95% CI: from 112 fewer to 81 more per 1000);
- higher levels of failure or recurrence: 11.2%% versus 2.5%; RD=87 more per 1000 (95% CI: from 21 more to 153 more per 1000); and
- higher levels of amplified resistance: 6.7% versus 0%; RD=67 more per 1000 (95% CI: from 32 more to 119 more per 1000).
The GDG judged the benefits of DCMZ to be moderate and the undesirable effects to be moderate compared with WHO-recommended longer regimens. The certainty of evidence was judged to be very low overall, with probably no important uncertainty in the values that people place on the outcomes. Within the category of moderate effects, the undesirable effects were considered of greater weight and had higher certainty associated with them – in particular for the amplification of drug resistance. The trial data suggest that drug resistance includes losing FQ in almost all patients who failed treatment. Hence, the GDG determined that the balance of health effects probably favours the WHO-recommended longer regimens.
Conclusion
The GDG suggested against the use of the DCMZ regimen in patients with FQ-susceptible RR-TB.
Summary of other evidence
Additional data reviewed by the GDG relevant to these PICO questions.
Resources required and cost–effectiveness
Additional data reviewed by the GDG relevant to these PICO questions were the estimates of the regimen prices provided by the GDF based on the most recent drug pricing included in the GDF online catalogue (50), as shown in Table 2.7.
Table 2.7. Regimen cost estimatesᵃ
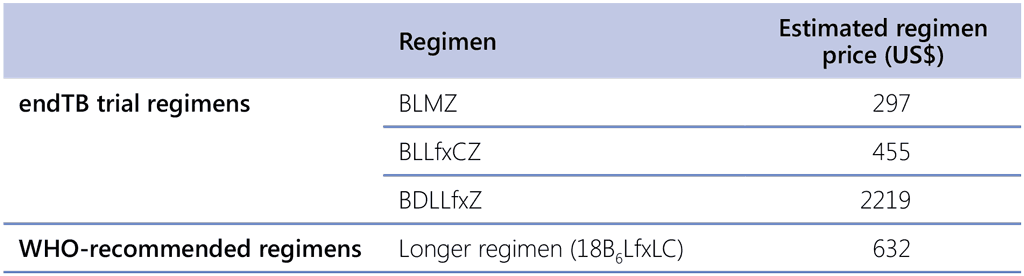
GDP: global domestic product; WHO: World Health Organization.
a Prices are based on the GDF online catalogue on [ADD DATE] (50).
As shown in Table 2.8, the GDG also considered the following example of country-specific patient-borne and health system costs over a 9-month span (excluding drug costs) (based on modeling analysis in Ryckman et al. 2024 (51). Costs may vary, depending on the composition of the regimen being used.
Table 2.8. Patient-borne and health system costs
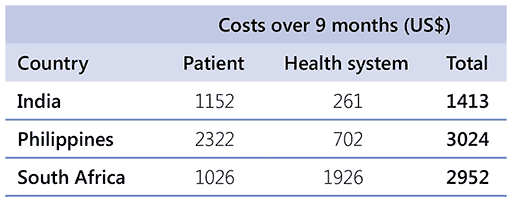
For all three recommended regimens, the drug prices were elicited from the Stop TB Partnership’s GDF; health system and patient costs in the three settings were estimated based on data from an economic modelling analysis (51), and extrapolated to the 9-month time period to account for the difference in the treatment durations. The estimated costs from the economic modelling analysis do not account for possible imprecision in the cost estimates; however, they do provide indirect data for other settings where the treatment regimen would be used. Therefore, the panel judged the certainty of evidence of required resources to be low.
The GDG noted that the drug prices for BLMZ and BLLfxCZ, are lower than the typical costs of the longer (18 month) regimens. In addition, shortening the treatment duration from 18 to 9 months would result in savings to both patients and the health system. Therefore, the GDG considered that – when compared with the currently recommended longer WHO regimens – the BLMZ and BLLfxCZ regimens would result in large savings. Although no research evidence on cost–effectiveness was available, the GDG discussed that, given the moderate net benefit and large cost savings with BLMZ and BLLfxCZ, a judgement of cost–effectiveness favouring the intervention is appropriate. This judgment is based on logical arguments that a cheaper regimen with better health outcomes will be cost-effective, although the exact savings are not known without such analyses.
For BDLLfxZ, the GDG noted that affordability will vary, depending on country (and resources available), health system differences and the population the regimen would be used for; accordingly, the GDG judged that costs would vary between moderate and large.
Delamanid is one of the major cost drivers in BDLLfxZ. With the drug being off-patent, prices may change with generic development. It was highlighted that a longer duration of treatment bears costs (especially for patients and families but also the health system) and some estimates were available and discussed by the group. Considering these costs together with the drug costs may attenuate some of the increased costs for the health system and lead to cost savings from the patient perspective. However, countries seeking to implement a specific treatment regimen typically focus on the drug cost. No evidence on cost–effectiveness was available for BDLLfxZ.
Equity, acceptability and feasibility
The panel considered the treatment duration and the ability to decentralize treatment (to enable access for remote, underserved settings and disadvantaged populations) to affect equity. Despite not being able to identify relevant research evidence, the panel used their collective experience to judge that there would probably be advantages associated with the use of BLMZ, BLLfxCZ and BDLLfxZ regimens, owing to their reduced complexity and shorter duration. In the case of BLMZ and BLLfxCZ, the panel also highlighted the possible positive effect of the overall lower cost. Therefore, the panel judged that use of the BLMZ, BLLfxCZ and BDLLfxZ regimens would probably increase equity.
In judging the acceptability of the regimens, the GDG considered patients and health care providers as key stakeholders. The GDG considered the regimen duration as critical with regards to acceptability, and therefore judged that the BLMZ, BLLfxCZ and BDLLfxZ regimens would probably be acceptable when compared with the longer 18-month regimens. Regarding BLLfxCZ, the GDG additionally noted that clofazimine may be less acceptable (e.g. because of skin discoloration), and noted 4% discontinuation due to clofazimine in the intervention arm in the trial. For the regimens BLLfxCZ and BDLLfxZ, there is also one additional drug (giving a total of 5 drugs) compared with the BLMZ regimen. Regarding BDLLfxZ, the GDG highlighted that delamanid requires taking medicines twice per day in this regimen, which may affect acceptability. Despite this, the GDG judged that BLLfxCZ and BDLLfxZ would probably be acceptable.
In judging the feasibility, the panel highlighted that the cost of BDLLfxZ (driven by delamanid) may affect feasibility for programme managers in particular.
Evidence to recommendations: considerations
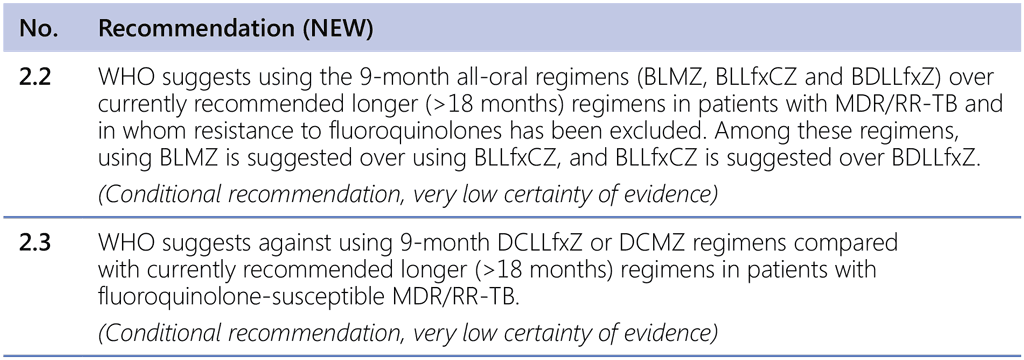
Based on the decisions taken during the review and the combination of assessments described above, WHO suggests using the 9-month all-oral regimens (BLMZ, BLLfxCZ and BDLLfxZ) over currently recommended longer (>18 months) regimens in patients with MDR/RR-TB and in whom resistance to FQ has been excluded. Also, WHO suggests against using 9-month DCLLfxZ or DCMZ regimens compared with currently recommended longer (>18 months) regimens in patients with FQ-susceptible MDR/RR-TB.
Among the newly recommended regimens, using BLMZ is suggested over using BLLfxCZ, and BLLfxCZ is suggested over BDLLfxZ. This ranking is based on an evaluation of all evidence and judgements made for individual sub-PICOs 2.1, 2.2 and 2.3, and deliberation by the panel. The panel was first asked to make judgements for each decision criterion about a comparative ranking the regimens (Table 2.9).
Table 2.9. Multiple comparisons of three recommended endTB trial regimensᵃ
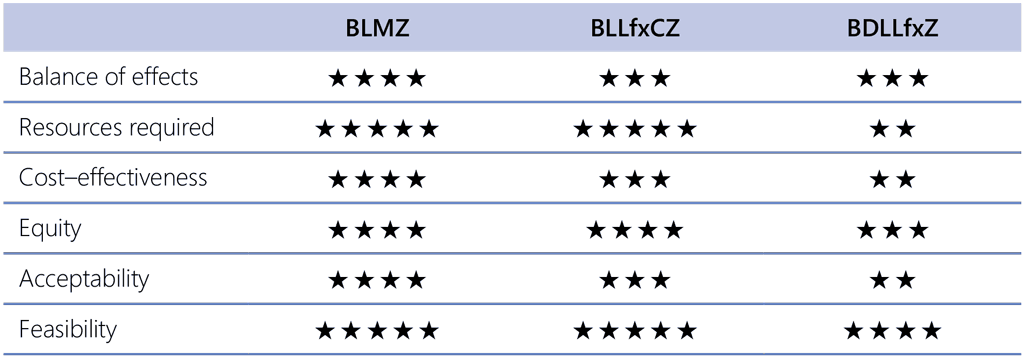
a The number of stars is used as a measure of ranking with a maximum of 5.
Following this, the panel was asked to deliberate about the ranking of the three regimens considering their previous judgements and all available evidence. The rationale for the ranking can be summarized as follows:
- BLMZ was preferred over BLLfxCZ and BDLLfxZ:
- BLMZ appeared preferable in terms of the balance of health effects compared with both BLLfxCZ and BDLLfxZ.
- BLMZ also has the lowest cost and pill burden, and appeared either preferable or equivalent for all other decision criteria.
- Therefore, BLMZ was deemed to be the preferred regimen between the three.
- BLLfxCZ was preferred over BDLLfxZ:
- BLLfxCZ, compared with BDLLfxZ, was deemed to have a similar but slightly preferable balance of health effects.
- BLLfxCZ also has a significantly lower cost and a lower pill burden than BDLLfxZ.
- The much greater cost of BDLLfxZ was judged to be likely to have negative effects on equity, acceptability and feasibility.
- Therefore, BLLfxCZ was deemed to be the preferrable over BDLLfxZ.
Subgroup considerations
Children and adolescents
Children and adolescents (aged 0–14 years) were excluded from the endTB trial; therefore, no analysis specific to this subgroup could be performed. Ten participants aged between 15 and 18 years were enrolled in the experimental arms (2 to BLMZ, 3 to BLLfxCZ, and 5 to BDLLfxZ). However, all medicines in the regimens have been used in children and have well-documented safety and efficacy profiles and sufficient PK/PD data. The GDG judged that it was appropriate to extrapolate from the efficacy data in adults from the endTB trial to children and adolescents.
As with adults, the BLMZ regimen is the preferred modified 9-month regimen for children, where its low pill burden and the availability of child-friendly formulations offers additional advantages. When these formulations are unavailable, practical guidance on adjusting adult formulations for children is provided in the operational handbook, to ensure that the lack of paediatric-specific formulations does not hinder treatment.
People living with HIV
The study included PLHIV regardless of their immunologic status. HIV was diagnosed in 98 (14.1%) people enrolled in the endTB trial, with 15 enrolled to BLMZ, 14 to BLLfxCZ, 17 to BDLLfxZ and 19 in the control arm. Provided that suppressive antiretroviral therapy is given, similar efficacy should be expected (stratified analyses largely supported this).
Pregnant and breastfeeding women
There were no data from the endTB trial on using the recommended regimens in pregnant and breastfeeding women. Other studies support that MDR/RR-TB can be managed during pregnancy with caution regarding the drugs used in BLMZ, BLLfxCZ, and BDLLfxZ (67, 68). Close monitoring and systematic collection of data from pregnant, breastfeeding and post-partum patients will offer valuable insights into treatment outcomes, thereby contributing to safer, evidence-based care for pregnant women with MDR/RR-TB.
Extrapulmonary TB
The endTB trial enrolled participants with extrapulmonary TB if they also had pulmonary TB; no specific analysis could be performed for participants with extrapulmonary TB. However, the GDG felt that extrapolation to extrapulmonary TB and other forms of TB was warranted except in cases involving severe forms of TB that may require special treatment arrangements and decisions, particularly TB involving the CNS, osteoarticular and disseminated forms of TB. Thus, the recommendation of the BLMZ, BLLfxCZ and BDLLfxZ regimens applies to people with pulmonary TB and all forms of extrapulmonary TB except for TB involving the CNS, and osteoarticular and disseminated forms of TB.
Other considerations
Several other patient groups were evaluated in the endTB trial. Excluded from enrolment were people with anaemia, uncorrectable electrolyte disorders, renal dysfunction, liver dysfunction AST, ALT or total bilirubin at least three times the upper limit of normal, with cardiac risk factors, a QTcF above 450 ms and other Grade 4 results. These groups of patients may still receive the regimens if the treating physician considers it the best option despite these possible contraindications.
Participants with diabetes, regardless of their HbA1c levels, could be enrolled. The panel found no evidence suggesting different conclusions for this group compared with the overall recommendations.
Implementation considerations
Patient selection
Eligibility for the three modified 9-month regimens is outlined under remarks on Recommendation 2.2. The regimens are considered for the treatment of patients with MDR/RR-TB in whom resistance to FQ has been excluded, and who cannot be offered any of the two recommended 6-month regimens (see consolidated operational handbook (69)).
The regimens are suitable for patients with pulmonary or all forms of extrapulmonary TB disease, except for TB involving the CNS, osteoarticular, or disseminated forms of TB with multiorgan involvement. Participants of all ages, including children and adolescents or PLHIV (regardless of CD4 count), diabetes (regardless of A1c), substance use disorders and mental illness could be enrolled.
Individuals with MDR/RR-TB who have had less than 1 month of previous exposure to any of the component medicines of the regimen (apart from PZA, where prior exposure is permitted; and quinolones, where resistance should be excluded), are eligible for treatment with these regimens. Additionally, the treatment programme may enrol children and adolescents who do not have bacteriological confirmation of TB or defined resistance patterns but are deemed to have a high likelihood of MDR/RR-TB, based on clinical signs and symptoms of TB and a history of contact with a confirmed MDR/RR-TB patient.
Drug susceptibility testing
A WHO-recommended rapid molecular test to confirm FQ susceptibility should be conducted before starting the treatment with the modified 9-month regimens. In settings where DST for other drugs in the regimen can be done and resistance to any of the component medicines of the regimen (apart from PZA, discussed separately below) is confirmed, the regimens should not be used.
The endTB trial data suggested reduced efficacy among patients with PZA resistance. However, the best estimates suggested higher success rates than with the longer regimen, even in cases of PZA resistance. Therefore, the GDG suggested that PZA can be dropped from the modified 9-month regimens if resistance to PZA is reliably confirmed or if there are PZA-associated AEs. However, if PZA is discontinued, the rest of the regimen should continue as prescribed. In settings where PZA testing is not widely available, PZA should be maintained unless there are PZA-related AEs.
Adverse events and drug–drug interactions
For patients on treatment with modified 9-month regimens, it is essential to undertake active TB drug-safety monitoring and management for close monitoring and adequately managing AEs and preventing complications from drug–drug interactions.
An important AE in patients using the modified 9-month regimens is hepatoxicity in relation to PZA. In the endTB trial, screening for elevation of liver enzymes was performed monthly throughout treatment, regardless of symptoms. Elevation in liver enzymes, with or without accompanying symptoms, occurred frequently during treatment. Grade 3 hepatotoxicity was defined in the trial as ALT (SGPT) or AST (SGOT) levels greater than five times but less than or equal to 10 times the upper limit of normal. Specifically, transient Grade 3 or higher hepatotoxicity occurred in 18% of patients in BLMZ, 16% in BLLfxCZ and 8.7% in BDLLfxZ in the safety population of the endTB trial. Imprecision in these estimates meant that it was not possible to draw any firm conclusions about differences between regimens. During the trial, suspension of PZA was recommended when liver enzyme levels exceeded five times the upper limit of the upper limit normal (5×ULN). PZA was permanently discontinued in an average of 17% of patients, with no significant differences among the three regimens. Most patients receiving the modified 9-month regimens received 39 weeks of PZA, and patients who permanently discontinued PZA received the drug for between 85 and 112 days, again with minimal variation between the regimens.
Regimen composition, dosing of component medicines and frequency
The short names of the regimens with one-letter abbreviations for the drugs, the three-letter abbreviations and compositions of the modified 9-month regimens are given in Table 2.10. All of the modified 9-month regimens have bedaquiline, linezolid, pyrazinamide and a fluoroquinolone as core, with one or two other additional drugs.
Table 2.10. The composition of the three modified 9-month regimens
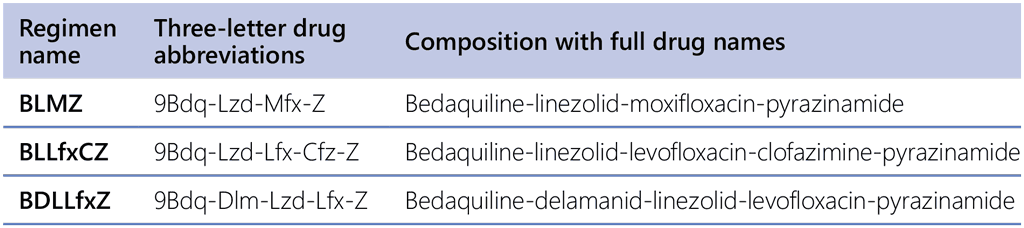
The dosing for linezolid and bedaquiline in these regimens deviates slightly from the standard dosing used in other treatment regimens:
- in the trial participants received linezolid at 600 mg once daily for 16 weeks then randomized to either reduced dose of 300 mg once daily or 600 mg three times a week until the end of treatment, outcomes appeared to be similar for both options;
- bedaquiline was dosed at 400 mg daily for the first 2 weeks, followed by 200 mg three times a week for the full 9-month period.
Both options for linezolid dosing strategy can be used. The alternative daily dosing of bedaquiline was not used in the modified 9-month regimens; however, it is considered an equivalent option that may simplify treatment for the patient by requiring the same number of pills every day, streamlining the dosing schedule. Dosing of the other drugs in the modified 9-month regimen follows the standardized weight-based dosing of medicines used in MDR/RR-TB regimens, for adults and children.
Regimen duration, extension and discontinuation
In general, the individual drugs in the modified 9-month regimens are all used for the full 9-month duration. All three endTB trial regimens were stopped at month 9 without an option for extending the duration. Discontinuation of either pyrazinamide or linezolid due to adverse events may be considered, and the regimen may continue with the remaining drugs. However, if more than a single drug needs to be discontinued, the regimen should be stopped and an alternative treatment started.
Where there is a lack of clinical or bacteriological response (e.g. culture remains positive or reverts positive at month 4 or beyond), there should be an investigation for a possible undiagnosed or acquired drug resistance.
Missing doses and treatment interruptions
Making up for missed doses follows routine TB practice when accumulative interruption of all medicines in the regimen exceeds 7 days but is less than a month.
Care and support
Treatment administration coupled with patient support can boost adherence and ensure optimal drug effectiveness and safety of patients on treatment. Measures to support patient adherence (e.g. by facilitating patient visits to health care facilities or home visits by health care staff, or by using digital technologies for daily communication) may be important to retain patients on treatment, even when a regimen is comparatively short. WHO recommendations on care and support are discussed in Chapter 3.
32 Extensive (or advanced) pulmonary TB disease is defined as the presence of bilateral cavitary disease or extensive parenchymal damage on chest radiography. In children aged below 15 years, advanced disease is usually defined by the presence of cavities or bilateral disease on chest radiography.
33 Severe extrapulmonary TB is defined as presence of miliary TB, TB meningitis, osteoarticular or pericardial TB. In children aged below 15 years, extrapulmonary forms of disease other than lymphadenopathy (peripheral nodes or isolated mediastinal mass without compression) are considered to be severe.
34 The three medicines included in Group A used for classification of second-line medicines are bedaquiline, fluoroquinolones and linezolid.
35 Note that while unadjusted effect estimates suggest a small reduction in levels of death, the adjusted effect estimates suggest a small increase in levels of death.
36 See the list of high-confidence resistance-conferring mutations in the WHO guide on the use of next-generation sequencing technologies, WHO (2018) (63).

 Feedback
Feedback